General Terms in Thermodynamics
General Terms in Thermodynamics: Overview
This topic covers concepts, such as, Thermodynamic System,System, Surroundings and Boundaries,General Terms in Thermodynamics etc.
Important Questions on General Terms in Thermodynamics
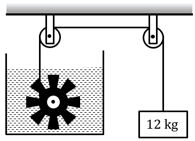
The figure shows a peddle wheel couple to a mass of through fixed frictionless pulley. The paddle is immersed in a liquid of heat capacity kept in an adiabatic container. Consider a time interval in which the block falls slowly through.
(a) How much heat is given to the liquid ?
(b) How much work is done on the liquid?
(c) Calculate the rise in the temperature of the liquid neglecting the heat capacity of the container and the paddle
.
Time taken to heat water upto a temperature of (from room temperature) is and time taken to heat mustard oil (of same mass and at room temperature) upto a temperature of is , then (given mustard oil has smaller heat capacity)
What is the thermodynamic system?
For the process to occur under adiabatic conditions, the correct condition is:
Which is not the microscopic variable of the system?
What are the macroscopic variables of a thermal system?
What is an isolated system. Give its one example.
The real or imaginary surface that separates the system from its surroundings is called _____.
What are the constituents of a thermodynamic system?
Heat flows from _____ temperature to lower temperature.
In thermodynamics, work done by a system is the total quantity of energy that the system and its surroundings exchange within themselves.
A system is said to be an isolated system if
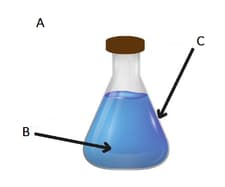
If we want to study the liquid inside the flask, we can define A, B and C as
What is a boundary for a thermodynamic system?
What do you understand by macroscopic parameters in thermodynamics?
Which of the following parameters are to be considered in determination of state of a thermodynamic system?
Explain System and Boundary.
Define surrounding in thermodynamic system physics
What Are The Types Of Boundaries In Thermodynamics